When you stop using fluoride toothpaste, you will lose all of the protective/strengthening tooth benefits that you were getting from fluoride. All of the strengthening and protect effects will be gone. It'll be almost as if you never used it!

If you're thinking about discontinuing your fluoridated toothpaste, you should carefully consider the benefits you'll be giving up. As a refresher, we will review all of the beneficial effects from using a fluoride-based dentifrice.
What fluoride does for your teeth:
Those are all of the effects and benefits from using a toothpaste with it. If you choose an all natural toothpaste such as charcoal or herbal then you'll be missing out on all of those perks. However there is one type of toothpaste that is a viable alternative to fluoride and that is hydroxyapatite.
Strengthens enamel
Fluoride is the only mineral in this world that can make your teeth stronger. It does this by converting the tooth mineral hydroxyapatite into fluorapatite by replacing the hydroxyl ion with fluorine.

This is a significant event because the enamel is composed of roughly 97% hydroxyapatite. That mineral is practically made of calcium and phosphates. It is what gives our teeth their structural rigidity and hardness.
Once the mineral transforms to fluorapatite, it gains new benefits:
Increased resistance to acid dissolution.
Decreases mineral solubility.
Increased stability of mineral structure.
Promotes remineralization to reverse cavities.
Essentially the fluoride makes the enamel stronger. The most quantifiable effect can be seen in the decrease of the critical pH level from 5.5 to 4.5 which means a stronger acid is needed to dissolve it.

If you have fluorapatite, you can consume more acidic foods or beverages without enamel demineralization. Of course you lose this trait if you stopped using the fluoridated toothpaste.
Repairs teeth by remineralization
If you eat too many sweets your teeth will start to demineralize, which is officially the first tooth decay stage. When this happens, the enamel will start to lose minerals.
Fortunately if you use a fluoride toothpaste you can reverse the process and repair the enamel via remineralization. It does so via two mechanisms
Decayed tooth structure more readily take up fluoride.
Calcium fluoride-like layer serves as a mineral reservoir of calcium and phosphate.
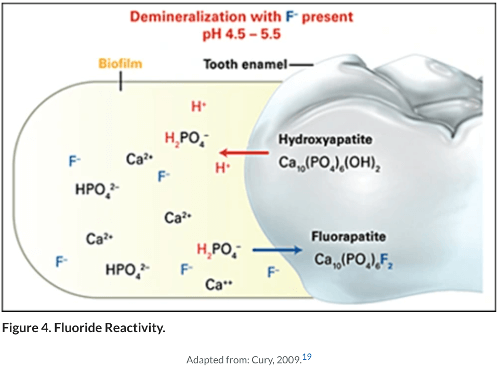
Studies have shown that intact enamel don't absorb as much fluoride as decayed or demineralized portions. When the tooth take in fluoride, it converts to fluorapatite which makes it stronger. The fluorapatite also initiates remineralization by actively pulling in calcium and phosphate from the saliva to repair the tooth.
Studies have also shown that topical application of fluoride such as in toothpaste will form a calcium fluoride-like layer over the tooth. This layer contains a lot of calcium and phosphates. When the layer dissolves during an acid attack, the minerals can be used to repair the tooth, hence acting as a reservoir.
Both of these mechanisms produce an effect which helps to repair the teeth when it is under an acidic or sweet attack. If you exchange your fluoride toothpaste for an all natural one, you will lose these effects.
Protects your teeth
The calcium fluoride-like layer which forms over the teeth from brushing with fluoride bestows a protective effect.
This layer serves as a sacrificial layer when subjected to an acid attack.
Dissolution of the layer releases phosphates which buffer the oral environment to make it less acidic.
This extra layer that forms over your enamel from brushing with said toothpaste serves as the first line of defense against cavities. It is akin to a barrier or you could call it a sacrificial layer because acid comes into contact with it first. Therefore, it will dissolve first before the enamel takes any damage. It essentially helps the mouth soak up some of the acid.
There is an additional benefit for when the calcium fluoride-like layer dissolves. It releases calcium and phosphates into the oral environment. What the extra phosphates do is help to buffer the mouth and make it less acidic. Essentially it helps to reduce the acidity and remove some of the acid and that helps to protect your teeth.
If you didn't know that phosphate can do that, it is actually one of the buffering systems that our saliva uses. The primary one is bicarbonate but phosphates will assist and help out in times of need.

Once again, you will have this protective effect only if you continue to a use a toothpaste with fluoride. You won't get this from a charcoal toothpaste.
Antibacterial properties
Unbeknownst to most, fluoride has antibacterial properties that interfere with the bacteria's sugar metabolism and its ability to take in sugar. The ultimate result is the bacteria starving to death.
The anti-bacterial mechanisms:one
Inhibits metabolism of glucose (glycolytic enzyme enolase activity)
Inhibits proton-extruding adenosine triphosphate (H+/ATPase) for molecular transport
Fluoride inhibits the activity of enolase, which is the enzyme that is responsible for forming phosphoenolpyruvate (PEP) in the glycolysis cycle. PEP is one of the intermediates in forming pyruvate that is used as an energy source. PEP is also used for transporting outside sugar into the bacteria.

Fluoride can also inhibit H+/ATPase, which is a transmembrane bound transporter of solutes across the bacterial cell membrane.
The importance of a functional H+/ATPase:
It actively pumps protons out of the cell.
Effectively maintains a proton gradient.
The proton gradient is involved in a lot of solute transportation such as sugar.
Ultimately, you'll be giving up these antibacterial effects if you no longer use a dentifrice with fluoride in it.
Decreases sensitivity
Uncontrolled teeth sensitivity can decrease your quality of life but did you know that fluoride has an anti-sensitivity effect?
Stannous fluoride can occlude open dentinal tubules.
Fluoride varnish with 5% sodium fluoride can reduce sensitivity.
Brushing with stannous fluoride based toothpaste has been shown to decrease sensitivity. The mechanism via how it works is by replugging all of the unclogged dentinal tubules. Those with chronic sensitivity tend to have open tubules or even enlarged ones.

Sodium fluoride in toothpaste may not be concentrated enough to elicit a desensitizing effect but the fluoride varnish version is. The varnish is 20x more concentrated than regular toothpaste so it immediately forms a thick layer of calcium fluoride over the teeth. This protective barrier shields the tooth from external stimuli.
Is there a fluoride alternative?
What happens when you stop using fluoride toothpaste is that you lose all of its beneficial effects. That means if you switch to an all natural toothpaste like charcoal, herbal, xylitol, or etc you may not get all of these anti-cavity effects.
However there is one exception and that is if you use a nano-hydroxyapatite toothpaste. This type of toothpaste is a valid alternative to fluoride because it possesses many of the same anti-cavity effects.

The reason being, the toothpaste is made of hydroxyapatite which is exactly what your teeth are made of. Research shows that the enamel is composed of roughly 96-97% hydroxyapatite while the dentin has 70% of it.
Beneficial effects from hydroxyapatite use:
Remineralizes teeth
Plaque control
Reduces teeth sensitivity
Whitens teeth
They're pretty similar to what fluoride can do if you categorize them broadly. However it does do one thing that fluoride doesn't and that is it can make your teeth appear whiter.
With all being said, we would like to emphasize that hydroxyapatite is a valid fluoride alternative. Studies have shown it to be effective as or at least equivalent to fluoride. Your dentist will not be offended nor sad if you switch to using it. The only downside is the cost because there is a price premium for it since it is so new.
Takeaway
Once you stop using fluoride toothpaste or switch to a non-fluoridated one, you'll lose all of the beneficial effects. That means you will no longer be able to strengthen, repair, and protect your teeth. You'll also lose the antibacterial and anti-sensitivity effects as well.
For that reason, our dentists in Long Island City do recommend continuing to use one since it is easily accessible and inexpensive. If you do want a fluoride-free toothpaste, we would recommend a nano-hydroxyapatite one. That has very similar anti-cavity effects as fluoride so it is a valid alternative choice if you didn't want too much fluoride in your life.
