Table of contents:
Overview
Fluoride (F) is a naturally occurring mineral that is found in water, soil, and air that has anti-cavity effects via strengthening and protecting teeth. All water has some amount of fluoride in it but is usually not enough to prevent cavities. However there are some natural water sources which do have a high level of it that is sufficient to prevent caries.

In fact, the discovery of the anti-cavity effects started from an investigation into mystery teeth staining that caused teeth to turn brownish. The phenomenon was independently observed by dentists Dr. Frederick McKay in Colorado in 1901 and in Naples in 1902 by Dr. J.M. Eager, who was stationed in Italy.
The locals called the tooth stains the "Colorado brown stain" but Dr McKay named it mottled enamel (Rocky Mountain Mottled Teeth). Despite the staining, these teeth were found to have a significantly lower rate of decay. The cause remained elusive until 1930 when they finally discovered that the staining and cavity resistance was due to excessive fluoride in water.
Then finally in 1945 the city of Grand Rapids, Michigan, became the first to have their water supply artificially fluoridated. That was the beginning of a 15 year trial of community water fluoridation that was launched in four cities.
Grand Rapids, Michigan, is paired with neighboring Muskegon
Newburgh, New York, with Kingston;
Evanston, with Oak Park
In Canada - Brantford, Ontario, with nearby Sarnia
The end result of this was water fluoridation being named one of the 10 greatest public health achievements in the 20th century. Now without further ado, let's dive into what fluoride does for your teeth.
Additional information: Timeline for community water fluoridation by CDC
Beneficial effects of fluoride on teeth
Fluoride is good for teeth because it can strengthen, repair, and protect them from tooth decay and acid dissolution. The cavities are most notably caused by bacteria processing the sugar that we eat. Aside from sweets, teeth can also weaken and demineralize if we consume an excessive amount of low pH foods or beverages.
Low pH foods:
Sour foods like acidic salad dressings
Sweet foods and drinks like juices
Acidic beverages like wine
Spicy foods
How fluoride helps teeth is by is preventing these adverse conditions via three broad mechanisms:
Inhibition of demineralization
Enhancement of remineralization
Inhibition of bacterial activity
These mechanisms are multifaceted but can be understood more easily when broken down further into perceived benefits. The scientific terms above may not fully translate into what it can do for you which is why we'll explain it in a different way!
Strengthens teeth
Fluoride can strengthen your teeth by making it more resistant to demineralization by converting some of the tooth minerals to fluorapatite.
Your teeth are primarily composed of the mineral hydroxyapatite, which gives it its strength, hardness, and rigidity. The molecular formula for it is Ca10(PO4)6(OH)2 which means that it is essentially made of calcium and phosphate. Your enamel actually consists of 97% hydroxyapatite by weight while the dentin has 70%.
The enamel can become strengthened when fluoride interacts with the hydroxyapatite:
Fluorine replaces the hydroxyl ion (OH).
Hydroxyapatite transforms into fluorapatite [Ca10(PO4)6F2].
Not all of it gets converted to fluorapatite because even in severely fluorosed teeth only 10% converts.
Although we call it fluorapatite it is technically a hydroxyapatite-fluorapatite compound.

Reasons why fluorapatite is superior:
Increased resistance to acid dissolution.
Decreases mineral solubility.
Increased stability of mineral structure.
Promotes remineralization to reverse cavities.
In other words, your enamel becomes a better version of itself when it becomes exposed to fluoride. It becomes stronger and more resilient with fluoride incorporated into its structure.
Critical pH is lowered by fluorapatite
Hydroxyapatite begins to dissolve under acidic conditions when the oral environment drops below the critical pH level (5.5). At that level, there is a net effect of teeth demineralization (loses minerals) over remineralization (gains minerals).

On the other hand, fluorapatite is more resistant to acid dissolution due to having a lower critical pH (4.5) than hydroxyapatite. In other words, having fluoride incorporated into your tooth mineral permits it to withstand more acidic environments. That is why we say it strengthens your teeth.
Repairs teeth - Remineralization
Fluoride can enhance remineralization by repairing teeth that have begun dissolving from cavities or acids. (Yes, remineralizing teeth is the equivalent of reversing cavities.)
Cavities and acidic conditions in the mouth can cause the teeth to dissolve or lose minerals, which is referred to as demineralization. However the process can be reversed with remineralization where the tooth regains the lost minerals.

Fluoride is able to help remineralize the teeth when it is applied topically from toothpastes, mouthwashes, and fluoride treatments.
Presence of fluoride promotes remineralization over demineralization.
Calcium fluoride-like layer which covers the enamel surface serves as a reservoir for calcium and phosphate.
Presence of fluoride influences demineralization and remineralization dynamics
In addition to fluorapatite being resistant to demineralization, the mere presence of fluoride not only inhibits it but shifts the equilibrium towards remineralization.
Studies have shown that intact tooth structure is NOT as reactive to fluoride when compared to demineralized ones. Therefore a tooth in distraught more readily takes up fluoride because it knows that it is in danger of acid dissolution. However a healthy tooth is not as inclined to take in F.

The significance of carious tooth structure taking in fluoride is that it accelerates remineralization. The fluoride will consequently draw in calcium and phosphates, thus effectively remineralizing the tooth by adding minerals back in.
Studies have shown that adding fluoride to a demineralizing environment will stop it. In fact, by increasing the amount of fluoride it starts to reverse it and begin remineralization. There was an exponential quantitative relationship between fluoride concentration and inhibition of demineralization or enhancement of remineralization.
Limitations to remineralization
Fluoride may help shift the dynamics towards remineralization under acidic environments but a limiting factor can be the quantity of calcium and phosphate in saliva.
There can be an overabundance of fluoride in your mouth but if there aren't enough minerals to remineralize the tooth, it will all be for naught. If you recall, hydroxyapatite is composed of the two minerals calcium and phosphate. Fluoride may assist in the process but you still need those two minerals in order to repair the tooth!
That is why it is important to have a nutritious diet that is full of minerals that can strengthen and repair the enamel. You don't only have to maintain good oral hygiene by brushing and flossing but you also have to eat well.
Calcium fluoride-like layer is a mineral reservoir
Topical applications of fluoride such as brushing with a fluoridated toothpaste will form a layer of calcium fluoride-like material over the tooth surface. It is not pure calcium fluoride since phosphate is also adsorbed into it.
Essentially this layer collects the minerals calcium and phosphate. When the tooth is subjected to acid challenges, it will dissolve and release the bound minerals. Your tooth could then reuse these very same minerals to remineralize the tooth, thus effectively serving as a mineral reservoir.
That is very helpful for repairing the tooth because you don't have to rely on just what is available in the saliva at that moment. It is an extra reserve for minerals that will repair the tooth.
Protects teeth
Fluoride can help protect the teeth via the calcium fluoride-like layer which is formed over the enamel via topical application. In other words, it can help prevent cavities.
How this calcium fluoride layer protects the teeth:
It dissolves first under acidic challenges thus serving as a sacrificial layer.
The dissolved layer releases phosphates, which can help buffer the acid.
Sacrificial layer that dissolves first
When the tooth is subjected to acid challenges, the calcium fluoride-like layer will dissolve first. It will do so more readily than the enamel layer which it is protecting.
Essentially it is an extra layer or barrier that covers your teeth, which acid has to dissolve through first before it can reach the enamel. Acidic attacks will have to work twice as hard to demineralize your tooth.
Releases minerals that buffers acidic environment
When the calcium fluoride-like layer dissolves, it will release all of the bound minerals, fluoride, calcium, and phosphate. They can be used to repair the tooth via remineralization or buffer the acidic environment to prevent further dissolution.
The primary buffering mechanism in saliva is the bicarbonate system. However under acidic challenges, the demineralized phosphates can also assist in buffering the oral environment. The acidic protons (H+) will combine with the phosphates (PO4) to form a weaker acid that is less harmful.

The uptake of the protons by phosphates helps in de-acidifying the environment by raising the pH level. Having a less acidic environment will halt demineralization and help shift it towards remineralization.
Anti-bacterial
The antibacterial effects of fluoride have been well established but there is a lack of consensus on how much it contributes in preventing cavities.
Fluoride effects on bacteria:
Inhibits metabolism of glucose (glycolytic enzyme enolase activity).
Inhibits proton-extruding adenosine triphosphate (H+/ATPase) for molecular transport.
Essentially what the fluoride does is interfere with the bacteria's ability to process sugar and take in sugar from the mouth.
Inhibits enolase (glycolytic enzyme)
Fluoride inhibits the activity of enolase, which is the enzyme that is responsible for forming phosphoenolpyruvate (PEP) in the glycolysis cycle. PEP is one of the intermediates in forming pyruvate that is used as an energy source. PEP is also used for transporting outside sugar into the bacteria.

Mechanism of antibacterial effect:
Enolase converts 2-phosphoglycerate to phosphoenolpyruvate (PEP). This is important because this is the second to last step for the conversion of glucose to pyruvate.
PEP is used as an energy source for the phosphotransferase system (PTS). The PTS is used by bacteria to transport sugar from outside of the cell to inside.

Essentially fluoride blocks the conversion of glucose to pyruvate in the second to last step by preventing PEP conversion. That effectively puts a halt in the carbohydrate metabolism of bacteria.
The absence of PEP is also problematic in that bacteria use it in the phosphotransferase system to take sugar into the cell. PEP is the energy source that they use to bring in their food source (glucose). With the PTS out of commission, the bacteria will practically starve.
Inhibits H+/ATPase
Fluoride can inhibit H+/ATPase, which is the transmembrane bound transporter of solutes across the bacterial cell membrane. It is normally powered by ATP as an energy source.
The importance of a functional H+/ATPase:
It actively pumps protons out of the cell.
Effectively maintains a proton gradient.
The proton gradient is involved in a lot of solute transportation such as sugar.
In addition to inhibiting the pump, fluoride will also diffuse into the cell as HF but dissociate into H+ and F-. That only worsens the already poor proton gradient. The purpose of the H+/ATPase is to pump protons out of the cell by deacidifying it.
However the fluoride is bringing protons into the cell by acidifying it. In addition to that, it prevents the H+/ATPase from working. The ultimate effect is that the cell is unable to transport necessary solutes into and out of the cell since there is no concentration gradient.
Anti-sensitivity
Certain types of fluoride or sufficient concentrations of it can reduce teeth sensitivity.
Stannous fluoride is a desensitizing agent
High concentrations of sodium fluoride is a teeth desensitizing treatment
Stannous fluoride in toothpaste can desensitize teeth
Stannous fluoride (SnF2) in toothpaste not only prevents cavities but also possesses desensitizing properties. Studies have shown that use of the toothpaste decreased dentinal hypersensitivity after the 4 and 8 week mark when compared to a control.
Mechanism of stannous fluoride's anti-sensitivity effect:
SnF2 reduces sensitivity by occluding open dentinal tubules.
The occlusion was with a deposit consisting of tin, zinc, phosphate, and silicon.
Essentially this form of fluoride takes advantage of the tin (Sn) to form a complex that is capable of blocking open tubules. That protects the tooth nerve endings from being exposed to external stimuli such as cold. Chronic sufferers of dentinal hypersensitivity will typically have wide open tubules.

Fluoride varnish (5% NaF) is a desensitizing treatment
Unlike stannous fluoride, sodium fluoride in toothpaste does not desensitize teeth at least in the concentration that it comes in. However when it is applied to teeth in a much higher concentration such as in fluoride varnish (5%) it can reduce sensitivity.
Studies have shown that after 3 applications of fluoride varnish, it elicited an anti-sensitivity effect that gradually improved over the course of 8 weeks. That implies it is an effective long term strategy for treatment of hypersensitivity.
Fluoride varnish's anti-sensitivity mechanism:
High concentration of NaF induced the formation of a calcium fluoride-like (CaF2) layer.
The CaF2 layer penetrates into the dentinal tubules and occludes it.
To be clear, this effect only happens at high concentrations of NaF. The 0.25% of NaF that is commonly found in toothpaste is insufficient to elicit it. The fluoride varnish has 5% NaF, which is effectively 20x the concentration.
How does fluoride work?
Fluoride works in different ways to protect your teeth depending on whether it is firmly bound or loosely bound to the tooth. Both types of bound fluorides will protect the tooth from decay and make it more resistant to cavities.
The fluoride can be acquired either by taking it in systemically or having it applied topically:
Systemic intake. Drinking fluoridated water or taking fluoride supplements.
Topical application. Using fluoridated toothpaste and mouthwash or fluoride treatment at the dentist.
How firmly bound fluoride works
Fluoride can become firmly bound to the tooth when it replaces the hydroxyl group of hydroxyapatite. This reaction converts it into fluorapatite, which is a bigger, stronger, and more stable structure that is resistant to cavities and acid dissolution.
Fluorapatite can form under two conditions:
Fluoride that is taken in systemically while the tooth is still developing.
If fluoride is present during acidic challenges when the enamel is demineralizing.
The effects of having firmly bound fluoride in fluorapatite:
Reduction of tooth mineral solubility
Reduction of mineral diffusion from lesion
Interactions between tooth-bound and ambient fluoride
Essentially what transforming into fluorapatite does is, it raises the threshold before the tooth begins to demineralize. The fluoride helps the tooth maintain its structural integrity longer
Despite the benefits of having fluoride tightly bound in hydroxyapatite, research suggests that the cavity preventative effects from it may be limited. What may be more important for preventing tooth decay is the loosely bound fluoride.
How loosely bound fluoride works
Instead of being firmly bound, fluoride can also loosely bind to the surface of the tooth as a calcium fluoride-like (CaF2) layer. It is not a true layer of CaF2 since it is also contaminated with a lot of phosphates which are adsorbed to its surface. The phosphates actually stabilize it and prevent it from dissolving in the neutral pH of saliva.

It is often described as a layer that is calcium fluoride-like and not a fluorapatite layer. However when this layer is exposed to saliva for an extended period of time, a fluorapatite layer can form on top of the CaF2. The fluorapatite formation will restrict the dissolution of it more which in turn stabilizes it even further.
How the CaF2 layer forms:
When the tooth is exposed to a topical application of fluoride.
The oral environment is supersaturated with fluoride.
Most notably, this layer forms during the topical application of a concentrated 5% solution of fluoride varnish.
How the calcium fluoride-like layer works in helping teeth:
Acts as a sacrificial layer that dissolves first prior to the enamel doing so.
Serves as a reservoir for remineralizing minerals.
Sacrificial layer
Since this layer covers over the entire outer surface of the enamel, it receives the brunt of acid challenges. It takes all of the damage and will dissolve first, thus leaving the enamel layer underneath unscathed.
It serves as a sacrificial layer that protects the tooth beneath it. You can think of it as an extra barrier layer that acids and cavities have to dissolve through before it reaches the actual tooth.
Mineral reservoir for remineralization
The calcium fluoride-like layer is composed of all the minerals that the tooth needs to remineralize. Therefore when this layer dissolves, it will release all of the calcium, fluoride, and phosphates.
These minerals can then be used to simply remineralize any demineralized tooth structure. In addition to that it can also be used to buffer the oral environment to deacidify it. That will prevent further dissolution of the enamel.
Overall this additional layer is very helpful for your teeth to have. Not only does it help fight tooth decay, it can also assist in reducing teeth sensitivity. It will clog up open dentinal tubules as it encases the entire tooth to protect it. That effectively reduces dentinal hypersensitivity.
Fluoride side effects
Fluoride can be bad for you if it is used improperly such as taking in an excessive amount of it. There are teeth related and also non-teeth related side effects.
However, according to the CDC there has not been any convincing scientific evidence that links fluoridated water with any severe adverse health effects.
No risks associated with:
Cancer
Down syndrome
Heart disease
Osteoporosis
Bone fracture
Immune disorders
Low intelligence
Renal disorders
Alzheimer disease
With that being said, we will explore some of the alleged side effects from excessive fluoride.
Teeth related side effects of fluoride
The main adverse effect of excessive fluoride intake is dental fluorosis. This is a cosmetic condition where the teeth acquire an unsightly brown stain. In fact, it was this very same brown stain which prompted dentists to look into the cause of it and ending up discovering fluoride's anti-cavity effects.
This brown staining is also commonly referred to as mottled enamel. Since it was first discovered in Colorado, it was called the "colorado brown stain" by the locals.
The only adverse effect with this is the cosmetic appearance and nothing else because dentists found that these teeth were more resistant to decay. Therefore they may look ugly but they're actually stronger than non-fluorosed teeth.
How much fluoride will cause fluorosis?
According to studies, drinking water that is above 1.5 ppm has the potential to induce fluorosis. That is a level of fluoride which is much greater than what the recommendation for fluoridated water supplies are.
The current proposed level of water fluoridation by the FDA and the ADA is 0.7 ppm which is less than half of the level that will cause fluorosis.
How much fluoride will cause fluorosis?
According to studies, drinking water that is above 1.5 ppm has the potential to induce fluorosis. That is a level of fluoride which is much greater than what the recommendation for fluoridated water supplies are.
The current proposed level of water fluoridation by the FDA and the ADA is 0.7 ppm which is less than half of the level that will cause fluorosis.
Pros and cons
Here is a chart with an easy to understand benefits vs risks for using fluoride in your oral care products.
Pros for fluoride use:
Decreased risk of cavities
Strengthens enamel
Protects against acids
Remineralization
Anti-sensitivity
Antibacterial
Inexpensive
Easily accessible
Cons for fluoride use:
Colorado brown stain
Neurotoxicity
Fluoride overdose (Nausea, vomiting, abdominal pain, diarrhea, joint pain)
Skeletal fluorosis
Dental fluorosis
Thyroid problems
Consequences of not using fluoride
The major consequence of not using fluoride or any fluoride-based products is the increased risk of tooth decay. To reiterate, the entire story on water fluoridation started because scientists observed that there were certain populations that had less cavities. It took them nearly three decades of investigation to finally identify that the reduction of tooth decay was from fluoride.
Fluoride-free alternative that can protect against cavities
A fluoride-free toothpaste that can remineralize teeth would be nano-hydroxyapatite. These toothpastes can protect your teeth against cavities because as its name implies, it literally contains the same tooth mineral.
The effects of nano-hydroxyapatite toothpastes:
Repairs teeth by remineralization
Protects teeth against demineralization
Nano-hydroxyapatite can repair demineralized teeth by remineralizing them. The mechanism for how it does it is slightly different from fluoride. It is easier and simpler for nano-hydroxyapatite because it just needs to insert itself into the carious lesions. Since it contains all of the same minerals as your tooth, you can think of it as pre-made and ready to use.

In addition to the remineralization, nano-hydroxyapatite can also protect the teeth by forming a layer of hydroxyapatite covering the enamel. This characteristic is similar to how fluoride forms a calcium fluoride-like layer over the enamel. This layer will dissolve first under acid challenges and will consequently act as a reservoir for remineralization minerals.
An extra perk is that this extra hydroxyapatite layer will also prevent teeth sensitivity as well. It does so by occluding dentinal tubules, similar to how stannous fluoride does it. Overall, nano-hydroxyapatite is a valid fluoride-free alternative for remineralizing teeth. Studies have shown it to be equally as effective as fluoride in helping teeth prevent cavities.
Can you remineralize teeth naturally?
Yes, teeth can remineralize naturally because remineralization is a natural biological process. What these toothpastes with fluoride and nano-hydroxyapatite do is slow down demineralization and enhance remineralization. Essentially it pushes the equilibrium dynamics towards the teeth remineralizing instead of demineralizing.
Without the assistance of these two products, the process for remineralizing would just be a LOT slower. You will also run the risk of the dynamics tipping towards the demineralization side.
The question is do you want help or do you not want help? Having assistance is always more helpful than not having any if we can agree.
How to remineralize teeth naturally:
Eliminate all carbohydrate intake. This includes all sweets and even non-sweet tasting foods like bread and pasta.
Brush your teeth and floss them after every meal.
Drink plenty of water.
Eat a diet that is high in tooth minerals, calcium and phosphorus.
Consume sufficient vitamins such as A, C D, K, and potassium.
The rest is up to how diligent you are in following the protocol. Don't forget that even with natural remineralization, plenty of people still end up with cavity fillings. Nonetheless, the choice is yours to make.
Sources of fluoride
There are a variety of sources where your body and mouth can acquire fluoride. This naturally occurring mineral can be taken in either systemically or applied topically.
There are also different types of fluoride such as stannous vs sodium fluoride.
Systemic sources of fluoride
Systemic intake of fluoride is via ingestion and is most commonly through drinking water, foods, and fluoride supplements.
Fluoridated water
One of the easiest ways to get systemic fluoride into your system is by living in a community with a fluoridated water supply. You will naturally get it as long as you drink and use the tap water in your household.
According to the CDC, roughly 73% of the US population on public water systems have fluoridated water. Therefore there is a good chance that you already have this mineral in your tap water.
According to studies, the benefits of drinking fluoridated water will typically reduce cavities by about 27%. That is quite the accomplishment for something that you don't need to do anything extra for. Just drink water like you normally do and you'll receive all of the benefits.
How to check if your water is fluoridated if you use a public water supply:
Please visit the CDC website.
Input your state and then select your county.
It will tell you the water status of your county.
However if your water source is from a private well, you will need to hire a certified laboratory to test the water. Therefore the first thing you need to do is determine which type of water supply you have? Is it public or is it private?
Does bottled water have fluoride?
According to the American Dental Association, most bottled water have less than 0.3 ppm of fluoride. However it may vary depending on what water source that bottled of water uses. Remember that fluoride is a naturally occurring mineral in water sources so if the source has it then the bottled water will as well.
Unfortunately it isn't common practice to list the amount of fluoride on the labels of bottled water. This non-practice has been recognized by the community and there has been a recommendation of labeling it going forward. Whether or not this gets put into practice will require time.
Foods
Perhaps this may have come at a surprise for you but there are foods which naturally come with fluoride in them! According to Harvard University, it is a trace mineral that is naturally found in a variety of foods.
Coffee and brewed black tea
Shellfish, shrimp, and blue crabs
Oatmeal and raisins
Potatoes
Supplements
If you live in a community with fluoridated water, you've probably never had to take fluoride supplements. This type of treatment is usually only recommended for those with extremely high risk of tooth decay or live in a non-fluoridated community.
These supplements usually come in the form of tablets and lozenges that contain 1 mg, 0.5 mg, or 0.25 mg of sodium fluoride. They can be prescribed to those between the ages of 6-16 who are at high risk of cavities.
How to use fluoride supplements:
Chew or suck on the supplements for 1-2 minutes.
Swallow after the 1-2 minutes.
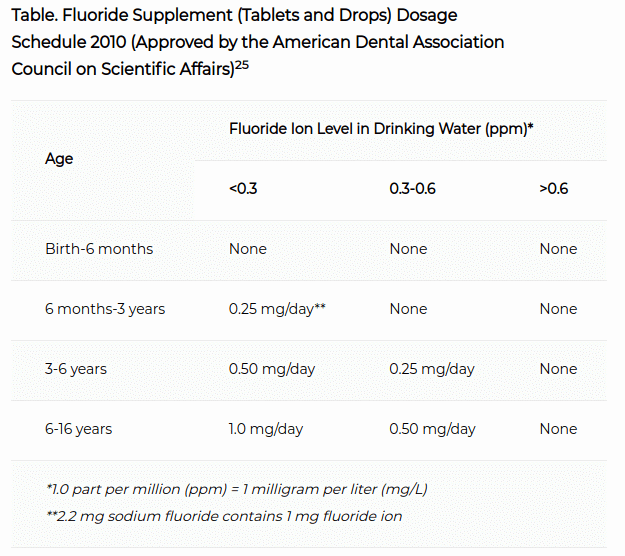
The table in the image above is a guideline by the American Dental Association about how much of the supplement to give. As you can see, it is dependent on how fluoridated the water supply is.
Topical sources of fluoride
Fluoride is easily accessible in a variety of oral care products such as toothpastes, mouthwashes, fluoride treatments at the dentist.
The fluoride can also come in different forms in all of these products:
Sodium fluoride (NaF)
Stannous fluoride (SnF2)
Sodium monofluorophosphate (SMFP or Na2PO3F)
Amine fluoride (not as common)
Toothpastes
Fluoridated toothpastes can come in any of the types of commonly used fluorides. This is one of the easiest ways to acquire fluoride for your teeth since you need to brush with a dentifrice twice a day. You might as well choose one that has fluoride in it.
All of the fluorides are effective in preventing cavities since they all contain the fluorine ion. There are minor differences among them though.
The stannous fluoride tends to cost more than the rest of them.
Potassium nitrate desensitizing toothpastes will often have the sodium fluoride paired with it due to compatibility.
The vast majority of the time, the type of fluoride that is used has to do with the stability and compatibility with the abrasives in it. Some of them aren't stable together so you may see a different type in it. However, the vast majority of people probably wouldn't even notice. They probably just want to know if it has any or it doesn't have any.
Mouthwashes
Not all mouthwashes contain fluoride but some of them do. You need to read the label carefully to see if it has it or not. It is very easy to use in that you just rinse with it as you normally would. You don't have to rinse in a special way with it.
Mouthwashes are an adjunct to brushing and flossing so don't go thinking you can just rinse and not do the latter! It is far more important to mechanically remove the the plaque via brushing your teeth and flossing them.

Examples of fluoridated mouthwashes:
ACT anticavity
Crest Pro-health complete
Listerine total care
Fluoride treatment
You can get a concentrated application of fluoride treatment at your dentist.
Prophy fluoride paste - This is the polishing paste at the end of your cleaning. You may not have known that it has fluoride but it does! It is typically 5x as concentrated as your toothpaste.
Fluoride varnish - This is a 5% concentration of NaF that is applied via a sticky gel. Afterwards you may not be able to eat for 2-6 hours depending on the brand.
APF gel - This comes in a gel or foam form that utilizes a plastic tray to apply it. You typically bite into the tray with the material for it to work.
The Verdict - What does fluoride do to your teeth?
The primary mechanisms associated with the role of fluoride in prevention of dental caries are inhibition of demineralization, enhancement of remineralization, and anti-bacterial effects. If you stop using fluoride toothpaste, you will lose all of these benefits.
Overall perceived benefits:
Strengthens enamel
Repairs decayed teeth
Protects against acid dissolution
Antibacterial
Desensitizing
Using fluoride can only help your teeth, granted that you use it as directed. However excessive amounts of fluoride can cause unwanted side effects such as fluorosis which is a brown stain on your teeth.
Nonetheless it is perfectly safe to use in moderation and won't cause any adverse effects. Drinking water with 0.7 ppm of fluoride and brushing with fluoridated toothpaste twice a day will only keep tooth decay away! It may even keep your dentist away if you brush more than that...
This article was brought to you by our dentists in Long Island City.
